May 5, 2021
How Teckro is to Clinical Trials what Fire was to Ancient Humans
Inspired by the words of a happy customer
Reading time

Gary Hughes
CEO & Co-Founder
TeckroA couple of weeks ago, I came across a rather wonderful comment about Teckro by a senior global trial manager who is using our technology to run a Phase III oncology trial. He said, “Teckro is great! It’s like man discovering fire.”
The quote was shared widely across our social media networks and had positive responses – both internally and externally, provoking much discussion. And it left me thinking about fire as a significant turning point in the history of early humans. So too, we think Teckro can have a similar impact for clinical trials, which was the motivation for us to create the company.
The discovery of fire is a defining feature of human intelligence, separating us from other animals. It’s not hard to imagine why fire was such a game-changer for humans – for one thing, fire meant that activities were no longer limited by daylight. Fire would have been useful for many things: light, warmth, scaring off woolly mammoths, cooking, creating more advanced hunting tools, etc. It was a huge step forward in humankind’s technological evolution.
Spark to Fire: Perfect Balance of Components Working Together
You may have heard of the “Fire Triangle.” For fire you need heat, oxygen and fuel, essential components to get the fire going. If you’ve ever run a campfire, you’ll know that it’s important to keep these three in balance or the fire will soon die out!
Now, let’s think about this idea of “components working together” in terms of clinical trials. Teckro has three main pillars that form the foundation for informed decisions that lead to faster, more efficient and safer clinical trials. They are: content, communication and insights.
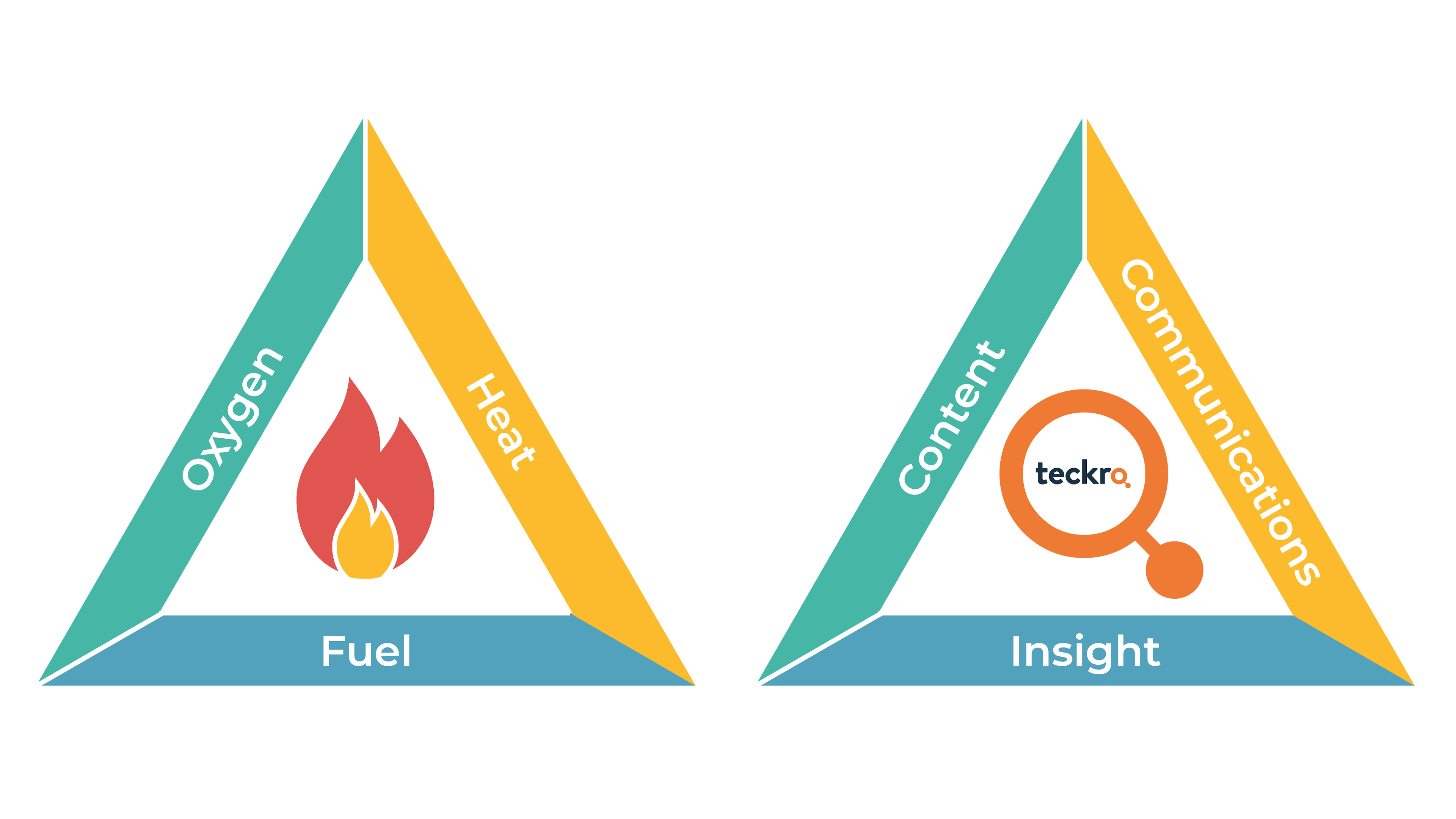
The first pillar is content.
When running clinical trials, all site staff need access to the latest version of the protocol for specific instruction and to avoid deviations. As a critical data source, the protocol, as well as other study documents, shouldn’t be a dusty paper binder tucked away or a portal that requires being behind a desk. Many site staff are not located in one place at the point of care, and accurate information should always be accessible from mobile and other digital devices for all site staff, as well as other study stakeholders. Teckro can help reduce the errors that stem from research personnel following outdated versions of the protocol or any other study document.
But, for as extensive as the protocol is with instructions, there will always be times when researchers need specific guidance or clarifications.
This leads us to the second pillar: communication.
Teckro directly connects research staff with study experts for real-time clarifications and guidance right from their mobile phone. Imagine the impact on decision making when a principal investigator can get guidance from a medical monitor in just a few minutes, rather than hours or days in the old model.
There is another level of communication where study teams can share proactive guidance, reminders or other alerts. This targeted communication is a big factor in helping sites to be more efficient, which can mean fewer errors, faster results and ultimately, more time with their patients.
In recent analysis, we saw patient accrual milestones were met three months early for a study on Teckro due to an increase in proactive guidance and alerts between study teams and site staff. This shows how real-time access to critical information and relevant communication supports all study stakeholders, including patients.
The third pillar is insights.
This is visibility that can help optimize trial performance and protect patient safety. Insights give a “bigger picture” of site engagement and communication effectiveness. It’s the difference between getting a clear picture of what sites are engaged with your protocol as you sip your coffee each morning and a rear-view mirror report that comes weeks too late to take any proactive action.
Search activity is a goldmine for insights to gauge not only activity but also identify potential safety risks for high profile terms. For example, one of our sponsors is using Teckro to gain insights into a known toxicity for their oncology trials. Knowing what sites are searching has allowed the study team to proactively manage and provide specific guidance when the term is being searched.
Teckro Fuels Clinical Trials
The trio – content, communication and insights – fuel what we can consider as the “fire triangle” for clinical trials. And just as you need a balance of components for fire, so too is the interdependent relationship between elements required for clinical trials. Teckro represents a breakthrough that is as revolutionary for the industry as the first human being who happened to work out that flames could be created by rubbing two sticks together (or by other means of creating friction), was for humankind’s development as a species over a million years ago.
The clinical trials industry is finally breaking out of the dark ages, entering an era of a mobile, digital, connected, up-to-date support system to help researchers make faster, more informed decisions. This leads to faster, safer, more efficient clinical trials – and most importantly life-changing treatments get to market faster. We created Teckro to be the game-changer for the industry, leaving the old ways behind.
Once humans found fire, they never went back. We have no doubt it will be the same for the clinical trial industry.